Efficacy and Safety of Voretigene Neparvovec in Japanese Patients with RPE65– Retinopathy: One-year Outcomes from the First Phase III Clinical Trial in Asia

Summary
A research group from the Laboratory of Visual Physiology, Division of Vision Research, National Institute of Sensory Organs (Director: Prof Kaoru Fujinami), the Department of Ophthalmology (Director: Prof Kunihiko Akiyama), and the Division of Vision Research, National Institute of Sensory Organs, NHO Tokyo Medical Center (Director: Prof Kazushige Tsunoda) at the National Hospital Organization (NHO) Tokyo Medical Centre, in collaboration with Novartis Pharma K.K. and the Japan Community Health Care Organization, has evaluated the efficacy and safety of the gene therapy voretigene neparvovec-rzyl (Luxturna®) for inherited retinal dystrophy caused by biallelic pathogenic RPE65 variants (RPE65-retinopathy) in Japanese patients, and has reported the findings.
This study represents the first Phase III clinical trial conducted in Asia, demonstrating sustained improvements over one year in visual functions such as dark-adapted light sensitivity and visual field, together with a favourable safety profile. On the basis of these results, Luxturna® (voretigene neparvovec) subsequently received regulatory approval and reimbursement in Japan, becoming the first gene replacement therapy approved in the field of ophthalmology in the country.
The findings of this study were published online in the international scientific journal Ophthalmology Science on 27 August 2025.
Preparatory Work Leading to the Clinical Trial
Since 2017, the Laboratory of Visual Physiology (Director: Prof Kaoru Fujinami) has advanced research into intractable inherited retinal dystrophies, including electrophysiological disease characterisation, genomic medicine, the introduction of AI-based analytical approaches, and clinical trial design. These initiatives have been pursued through international collaboration with more than 30 countries, and in partnership with governmental bodies, universities, research institutes, industry, and patient advocacy groups.
Key achievements include:
- Initiation and continuation of the first gene replacement therapy trials in Asia (RPE65-retinopathy and RPGR-retinopathy).
- Launch and continuation of the international post-marketing surveillance study of Luxturna®.
- Japan’s first clinical trial of a pharmaceutical therapy for Stargardt disease (tinlarebant; LBS-008).
- Establishment of national guidelines for genetic testing in inherited retinal dystrophies.
- Development of ACMG-based specifications for variant pathogenicity interpretation in genetic diagnosis.
- Formulation of guidelines for full-field stimulus testing (FST guidelines).
- Advancement of ophthalmic genomic medicine research and education through the IRD Genome Research Task Force.
- Support for the Stargardt disease patient organisation (Stargardt’s Connected).
As an extension of these efforts, this work has culminated in the successful implementation of the first approved gene replacement therapy in the field of ophthalmology in Japan.
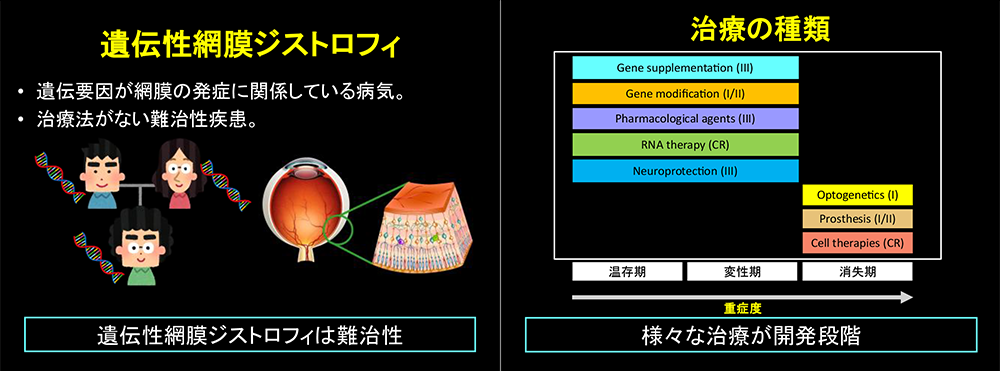
Background
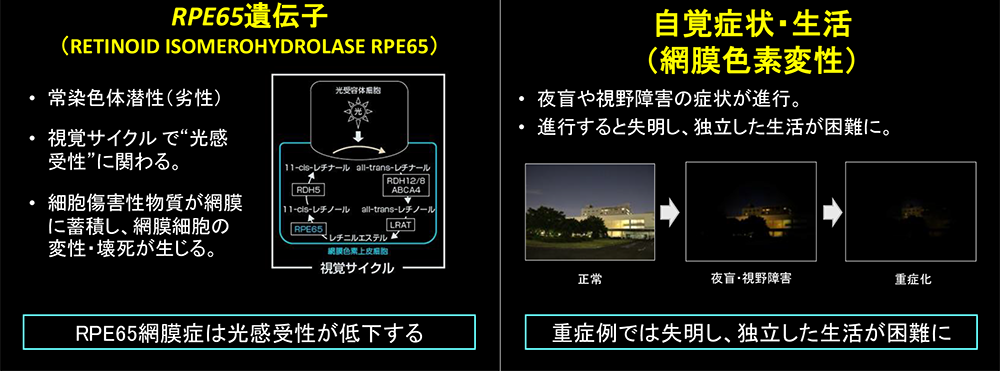
RPE65-retinopathy is a rare inherited disorder characterised by severe night blindness, progressive visual impairment, and constricted visual fields, caused by pathogenic variants in the RPE65 gene. The disease is progressive, and many patients experience severe difficulties in daily and social life by their twenties. Until recently, no effective treatment options had been available.
Voretigene neparvovec is the world’s first gene therapy for inherited retinal dystrophy. It utilises an adeno-associated viral (AAV) vector to deliver a copy of the RPE65 gene into retinal pigment epithelial cells, thereby restoring visual cycle function. While the therapy had already been approved in Europe and the United States, no clinical data were available for Japanese patients, rendering its use in Japan challenging.
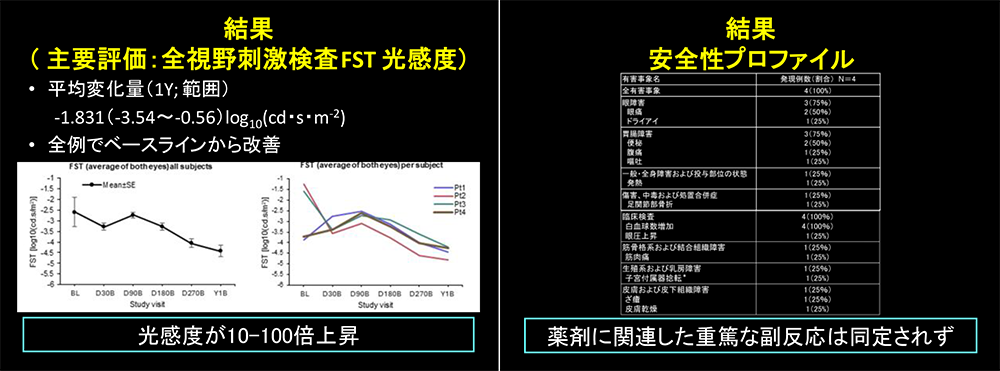
Results
- Participants:Four Japanese patients diagnosed with RPE65- retinopathy.
- Methods:Following vitrectomy, both eyes received subretinal administration of voretigene neparvovec(1.5×1011 vg/0.3 mL).
- Primary endpoint (FST):At one year, the mean binocular improvement was –1.83 log10(cd·s/m2), indicating enhanced scotopic visual function.
- Secondary endpoints:
- Visual field (Goldmann perimetry III4e):mean expansion of 427.8 degrees.
- Best-corrected visual acuity (logMAR BCVA):mean change of –0.033.
- Safety:All adverse events were mild to moderate in severity. One serious adverse event (ovarian cyst torsion) was judged unrelated to the study drug.
These findings confirm the efficacy and safety of this therapy in Japanese patients, establishing the foundation for the introduction of gene replacement therapy in ophthalmology in Japan. Long-term follow-up for up to five years is ongoing.
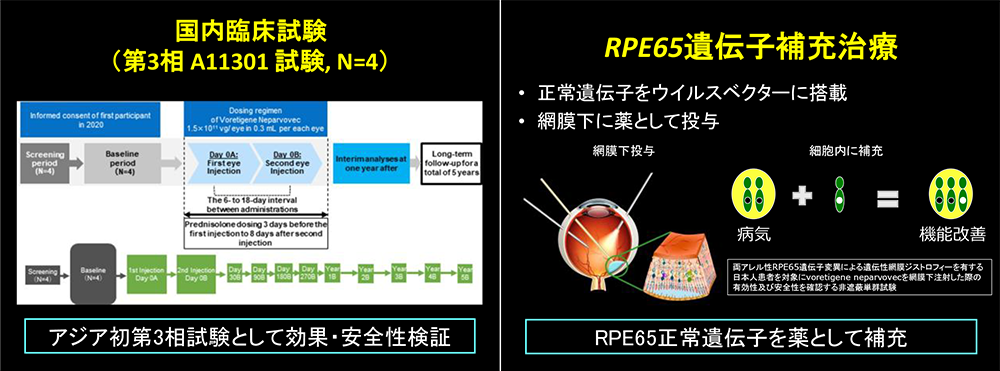
Future Perspectives
- Evaluation of the long-term durability of efficacy and safety through extended follow-up.
- Exploration of expanded indications, including paediatric patients and those with advanced disease.
- Translational research towards applications in other forms of inherited retinal dystrophy.
- Acceleration of the dissemination of gene therapy in rare disease fields, building upon international collaborative research.
Published Article
Journal:Ophthalmology Science
Title: Efficacy and Safety of Voretigene Neparvovec in RPE65-Associated Retinopathy: Results of a Phase 3 Trial in Japan
DOI:10.1016/j.xops.2025.100876
Publication date:27 August 2025
Authors:Kaoru Fujinami, Kunihiko Akiyama, Kazushige Tsunoda, Saori Ito, Noriko Seko, Shuichi Yamamoto
Funding
This study was supported by Novartis Pharma AG (Basel, Switzerland) (ClinicalTrials.gov identifier: NCT04516369).
Prof Kaoru Fujinami has also received funding from the Japan Society for the Promotion of Science (JSPS) KAKENHI, the National Hospital Organisation Network Research Fund, the Japan Agency for Medical Research and Development (AMED), the Foundation Fighting Blindness (USA), the National Institute for Health and Care Research (UK), and other funding bodies.
Glossary of Terms
- RPE65-retinopathy: A disorder caused by pathogenic variants in the RPE65 gene, leading to impaired vitamin A metabolism in photoreceptor/retinal pigment epithelial cells. It is characterised by severe night blindness, progressive visual impairment, and visual field loss.
- Gene therapy: A therapeutic approach in which a functional gene is introduced into cells to compensate for a defective or missing gene.
- FST (full-field light sensitivity threshold / full-field stimulus test): A diagnostic method that measures light sensitivity across the entire retina under dark-adapted conditions.
Contact Information
- For enquiries regarding the research:
Laboratory of Visual Physiology
National Hospital Organisztion Tokyo Medical Centre
Director: Prof Kaoru Fujinami
Tel:+81-3-3411-0111
Email: - For press/media enquiries:
National Hospital Organization Tokyo Medical Centre
General Affairs Division, Administrative Office
2-5-1 Higashigaoka, Meguro-ku, Tokyo 152-8902, Japan
Tel:+81-3-3411-0111

